
PRODUCT OVERVIEW

LEADING THE WAY AT
DECISIVE MOMENTS
What if the choices you make today,
could allow you to focus on
what matters most to you?

ULTRA REPRODUCIBLE
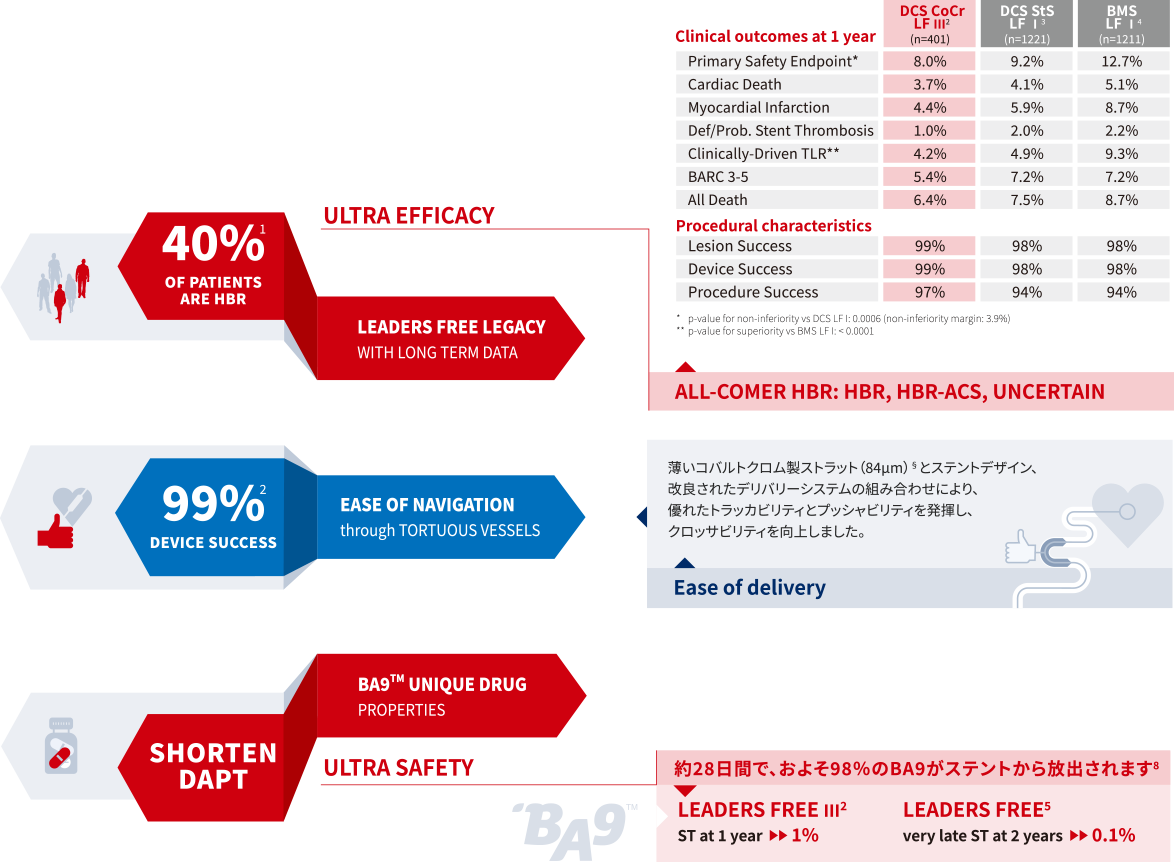
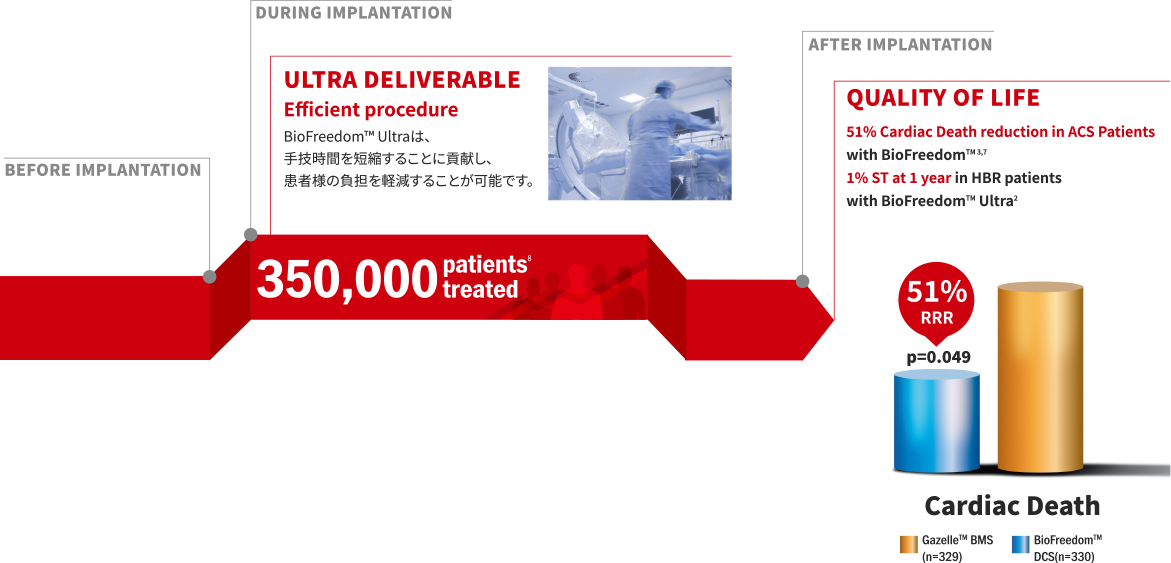
Positibve outcomes reproducible to all patients
3,000人のHBR患者を対象とした無作為化臨床試験
LEADERS FREE global clinical trial(I、II、III、Japan)では、
HBR患者の幅広い領域で治療効果の有意な改善が証明されています。
製品情報
PRODUCT INFORMATION
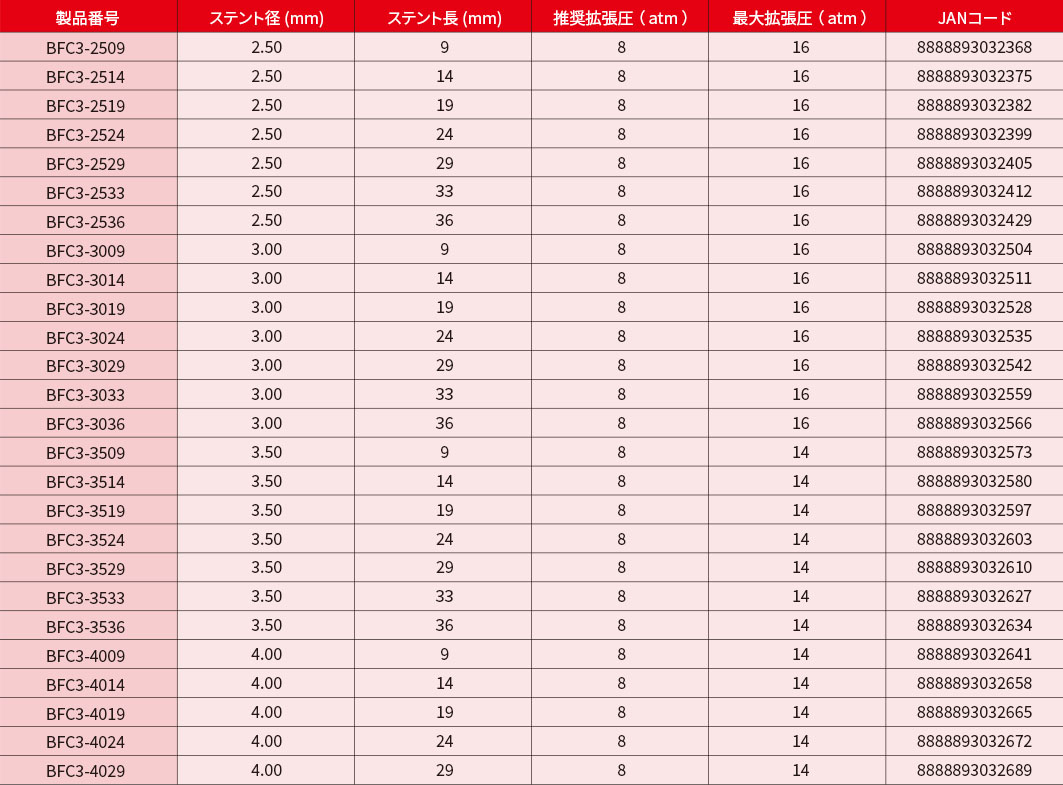
*印のついている製品は在庫をご確認の上、ご注文ください。
- ■ 販売名: BioFreedom Ultra 薬剤コーテッドステント
- ■ 医療機器承認番号:30400BZX00081000
BioFreedom™ Ultraは、CEマークの承認を受けています。
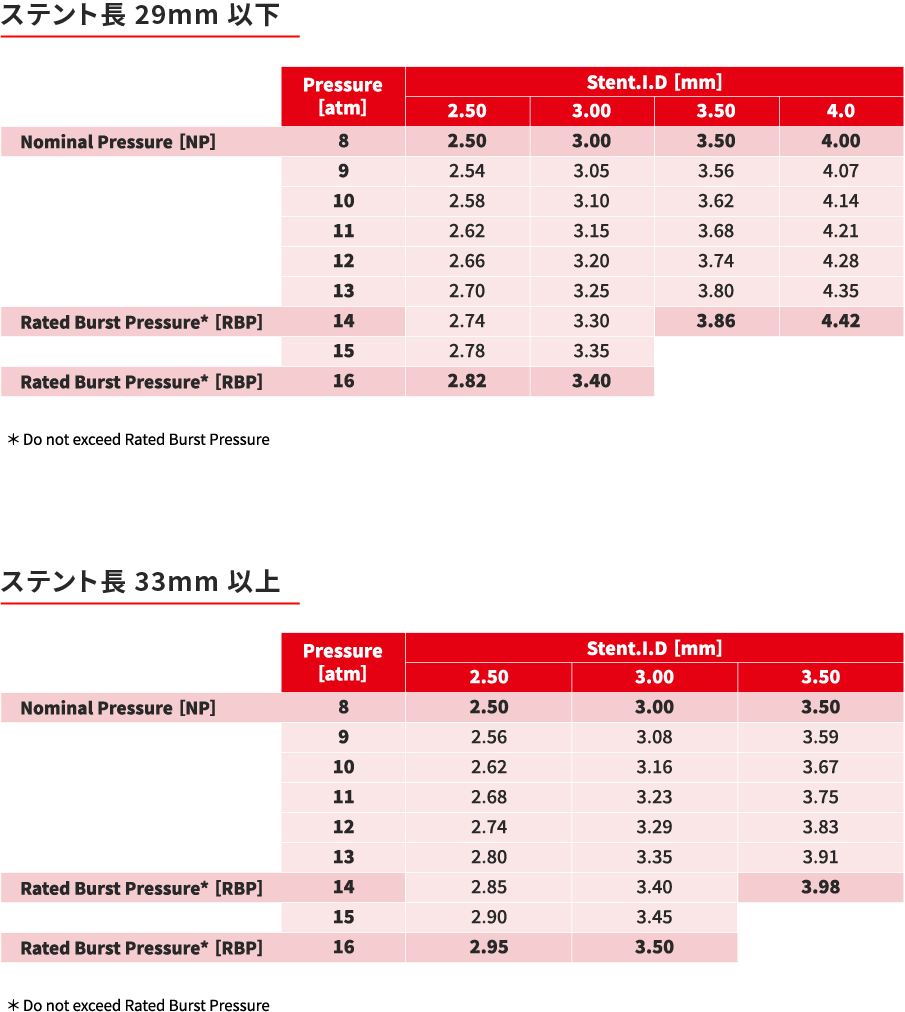
コンプライアンスチャート
COMPLIANCE CHART
![]()
BIOFREEDOM™
FAMILY CLINICAL PROGRAM
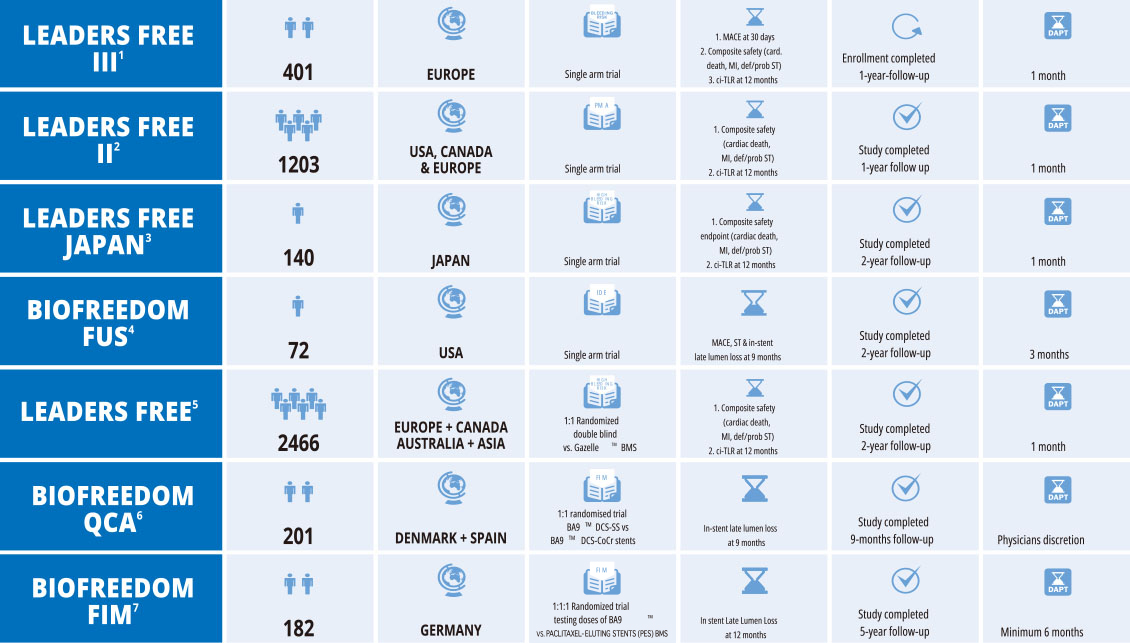
BioFreedom™ - Company Managed Trials
11855-000-EN_Rev.03d8
- 1. BioLimus-A9™ coated thin strut stents in High Bleeding Risk patients Oral abstract presentation Eberli R. PCR e-course 2020
- 2. Global Approach to High Bleeding Risk Patients With Polymer-Free Drug-Coated Coronary Stents: The LF II Study. M.W. Krucoff. Circ Cardiovasc Interv. 2020 Apr;13
- 3. ePoster Dr Saito Euro PCR 2017
- 4. Polymer-free Biolimus A9-coated stents in the treatment of de novo coronary lesions with short DAPT: 9-month angiographic and clinical follow-up of the prospective,
multicenter BioFreedom USA clinical trial Waksman R. et al CRM 18 (2017) 475–481 - 5. 2-Year Outcomes of High Bleeding Risk Patients After Polymer-Free Drug-Coated Stents. Garot P et al. JACC VOL.6 9, NO.2 , 2017
- 6. Main results of the BioFreedom QCA trial. Sabate M. PCR e-course 2020
- 7. Polymer-Free Biolimus A9-Coated Stents in the Treatment of De Novo Coronary Lesions 4- and 12-Month Angiographic Follow-Up and Final 5-Year Clinical Outcomes of the Prospective, Multicenter BioFreedom FIM Clinical Trial Costa R et al. J Am Coll Cardiol Intv 2016;9:51–64
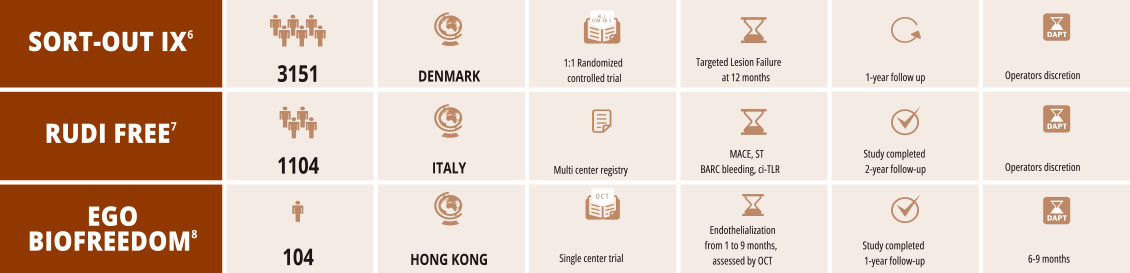
BioFreedom™ - Physician Managed Trials
with support from Biosensors International*
11855-000-EN_Rev.03d8
- * Biosensors has provided financial support for these PITs
- 6. Randomised Comparison of the Polymer-Free Biolimus-Coated BioFreedom Stent with the Ultrathin Strut Biodegradable Polymer Sirolimus-Eluting Stent in an All-comers Population
Treated with Percutaneous Coronary Intervention: The SORT OUT IX Trial. Published online Jensen L. 10.1161/CIRCULATIONAHA.119.0 - 7. Safety and efficacy of polymer-free Biolimus eluting stents in all-comer patients: The RUDI FREE study Sardella G, et al. EuroIn
- 8. Establishment of healing profile and neointimal transformation in the new polymer-free biolimus A9-coated coronary stent by lon
assessments: the EGO-BIOFREEDOM study Lee SWL et al. EuroIntervention. 2018 Sep 20;14(7):780-788
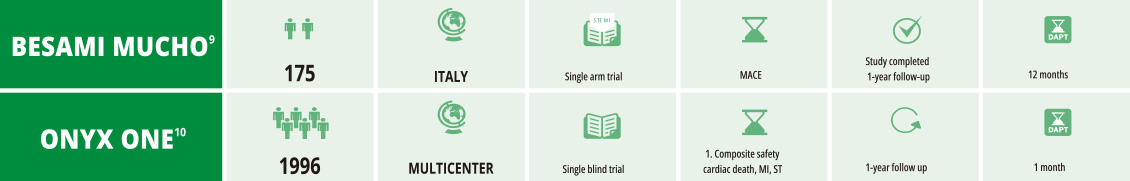
BioFreedom™ - Trials
with no Biosensors International involvement
11855-000-EN_Rev.03d8
- 9. Angiographic and clinical performance of polymer-free biolimus-eluting stent in patients with ST-segment elevation acute myocardial infarction
in a metropolitan public hospital: The BESAMI MUCHO study Gaspardone A Catheter Cardiovasc Interv.2017;00:1–8 - 10. Polymer-based or Polymer-free Stents in Patients at High Bleeding Risk Windecker S, et al. N Engl J Med. 12th February 2020; 1-11



