
LEADERS FREE
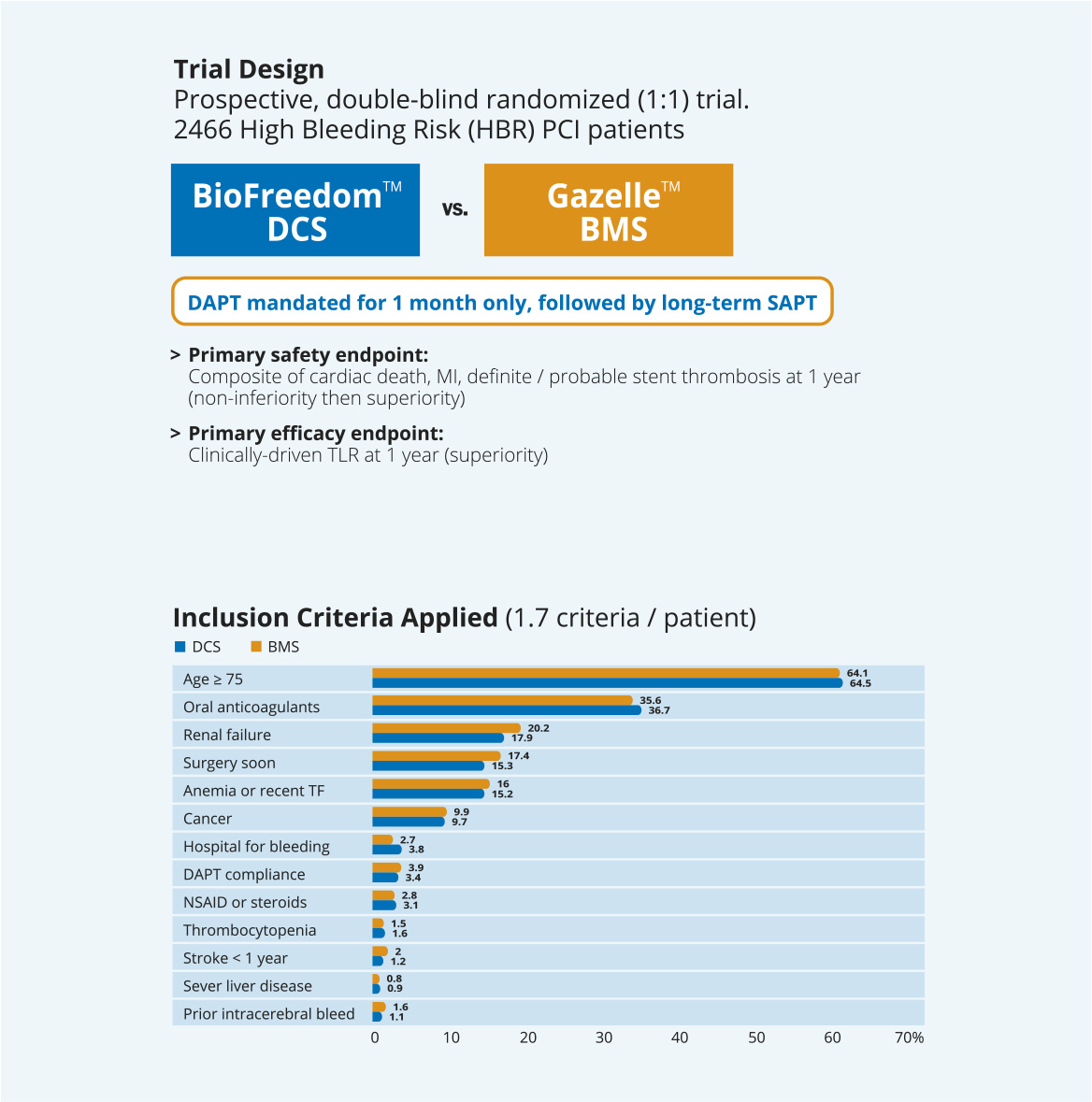
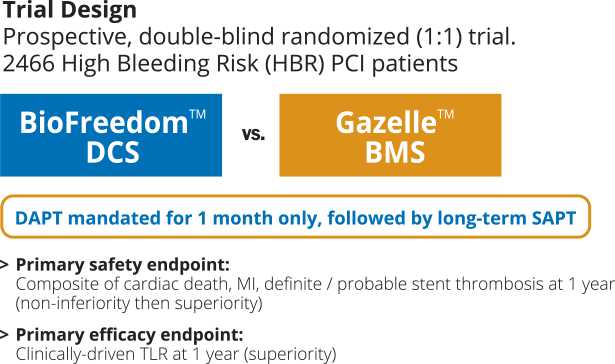
LEADERS FREEは、1ヶ月の2剤抗血小板療法を受けた出血リスクの高い患者2466例を対象に、BioFreedom™薬剤コーテッドステントとGazelle™ベアメタルステントを比較する前向き二重盲検無作為化(1:1)臨床試験です。
その結果、1ヶ月間のDAPTを併用した場合、1年後の安全性と有効性の主要評価項目に関して、BioFreedom™薬剤コーテッドステントがGazelle™ベアメタルステントより優れていることが実証されました。
BioFreedom™ Ultraは、BioFreedom™のテクノロジーを引き継いでいます。
- LEADERS FREE, at 1 year proved that BioFreedom™ is the only active stent with 1 month
DAPT that demonstrated significantly superior outcomes to
BMS in High Bleeding Risk Patients11. NEJM (October 2015) - LEADERS FREE 2 year follow-up maintains that BioFreedom™ and 1 month DAPT followed
by SAPT alone should be the treatment strategy of choice for
HBR patients undergoing PCI. JACC (January 2017)
- Polymer-free Biolimus-A9 coated thin strut stents for
patientsat high bleeding risk 1-year results from the LEADERSFREE III study - CCI(July 2021)
https://onlinelibrary.wiley.com/doi/full/10.1002/ccd.29869

Trial design and
unique HBR patients^
LEADERS FREE - 1 year follow-up:
BioFreedom™ is significantly
safer and more efficacious than a BMS
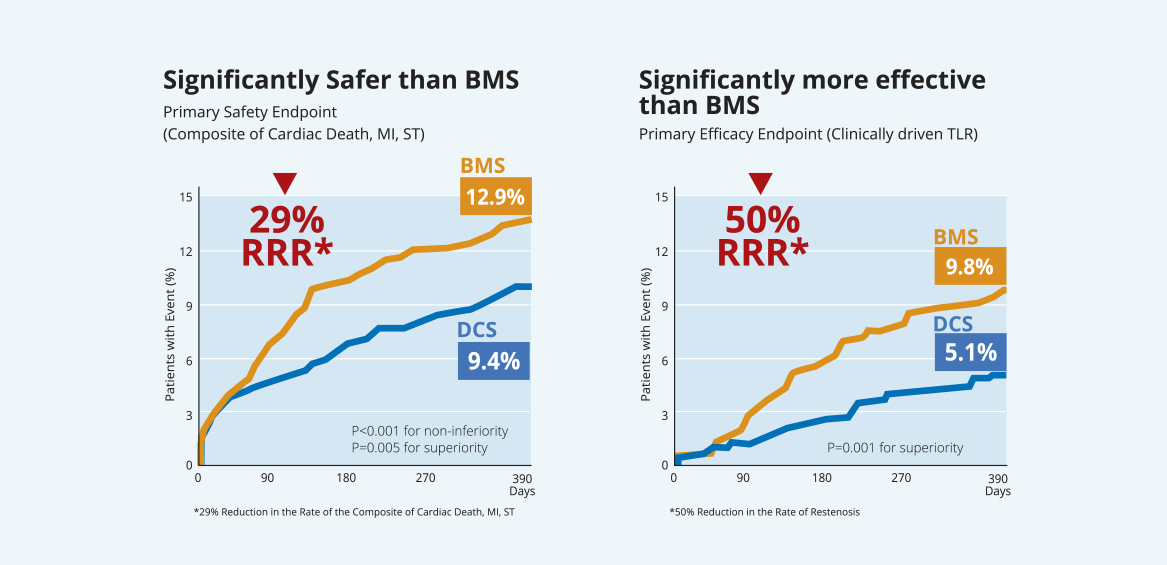
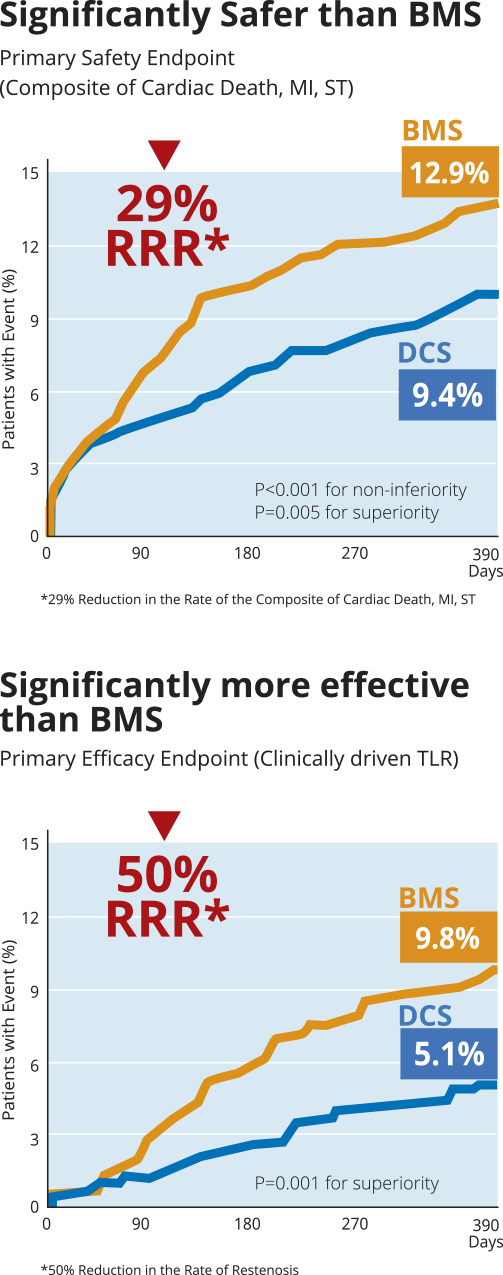
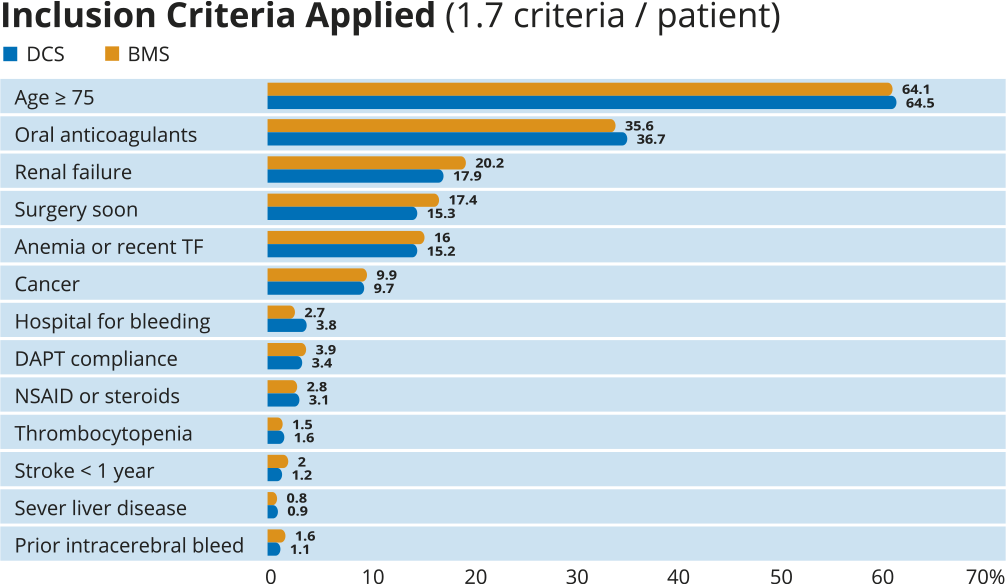
- LEADERS FREE is the first randomized clinical trial dedicated to HBR patients who received 1 month of DAPT followed by single antiplatelet therapy
- Such patients are often excluded from stent and drug trials, constitute a rapidly growing proportion of PCI candidates and suffer high event rates
- Together with an ultra short one-month only DAPT course, the use of BioFreedom™ (a Biolimus A9™ polymer and carrier free DCS) was both significantly safer and
more effective than a control BMS in HBR patients.
LEADERS FREE -
Pre-specified ACS subgroup -
Even greater safety and efficacy than
the general LF population
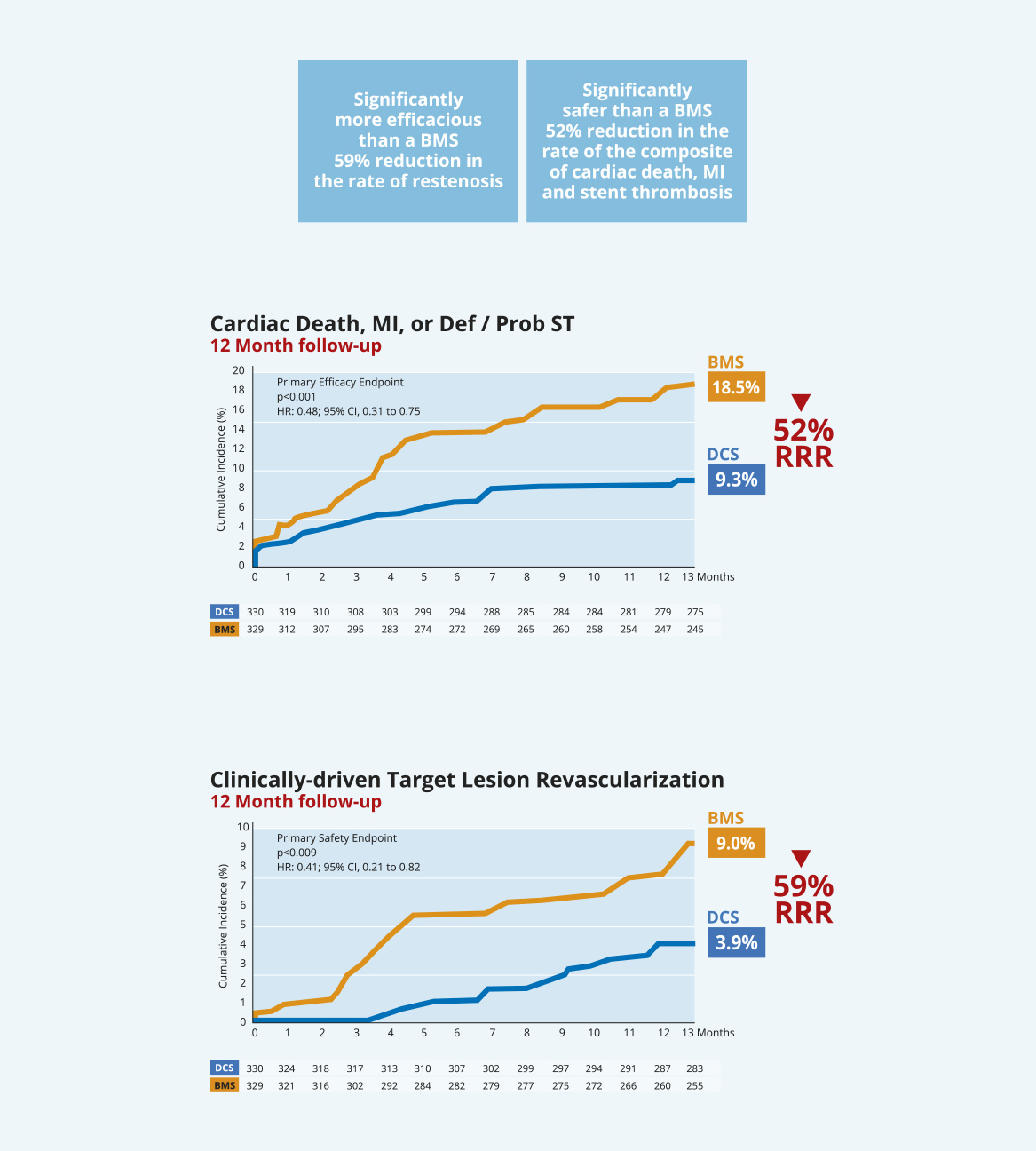
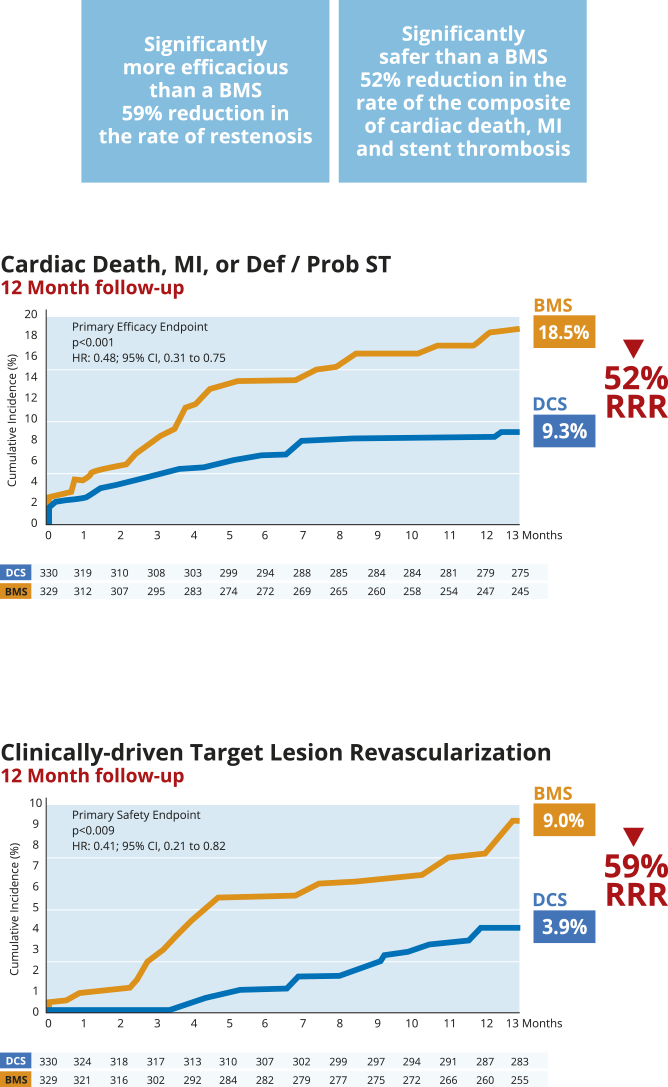
The pre-specified Acute Coronary Syndrome sub-group of the LEADERS FREE trial was presented as a late breaking clinical trial at Euro PCR 2016 by Dr Christoph K. Naber and published13.
This sub-group analysis reinforces the benefit of the BioFreedom™ DCS vs BMS in HBR patients. The improvement in safety and efficacy achieved with BioFreedom™ is even greater in the high risk HBR ACS patient population.
LEADERS FREE - 2 years:
Safety and Efficacy preserved
High Bleeding Risk patients:
safety and efficacy benefits of BioFreedom™ Drug-Coated Stent over BMS are
maintained at two years14.
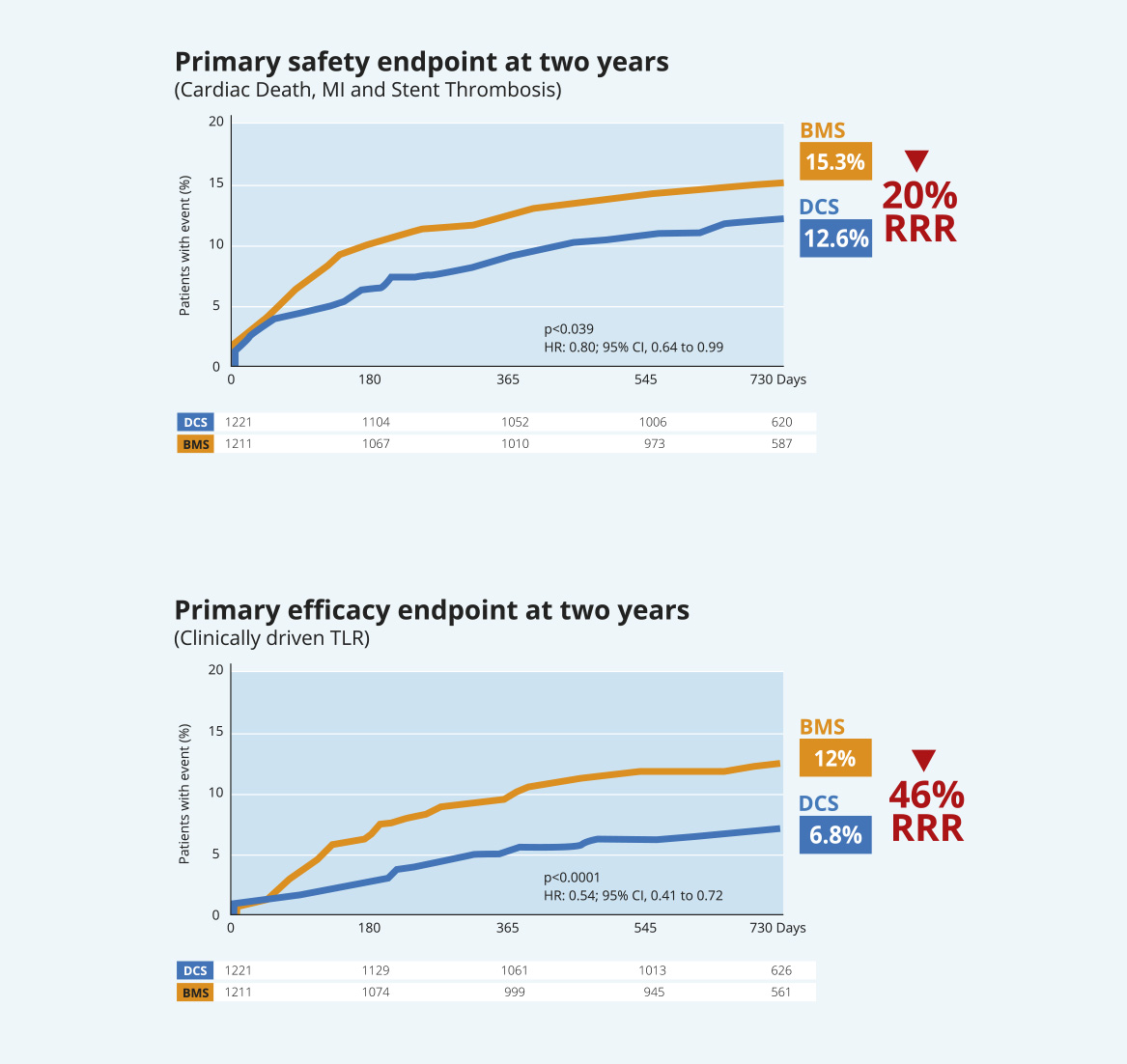
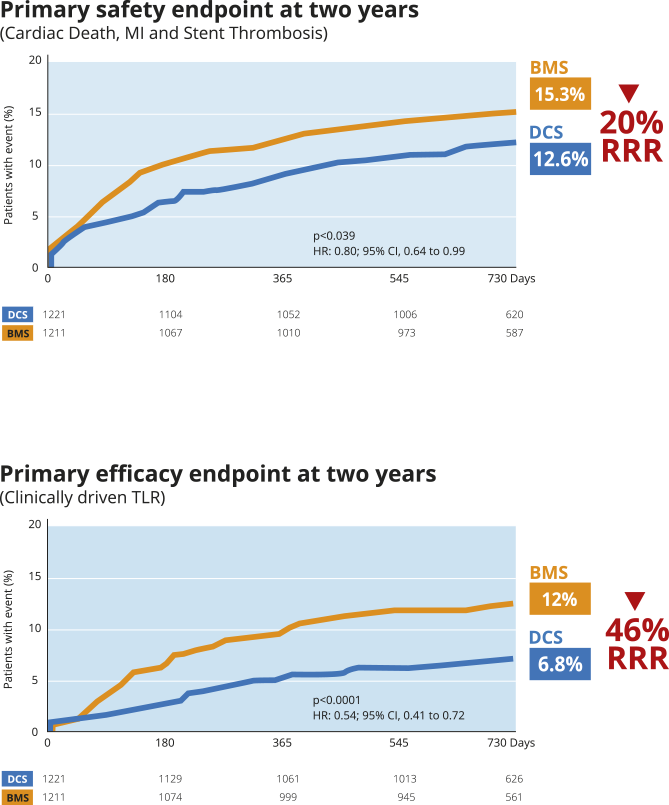
LEADERS FREE: Two-Year Outcomes of High Bleeding Risk Patients after Polymer-Free Drug-Coated stents14
BioFreedom™ is the only active stent with CE mark for ultra-short 1 month DAPT in High Bleeding Risk(HBR) patients,
supported by clinical data from a double-blind randomized controlled trial.
LEADERS FREE II -
Safety and Efficacy in
a North American population
Reproducibility of LEADERS FREE findings
- Safety of DCS with 30 day DAPT in HBR patients15
- Effectiveness of DCS with 30 day DAPT in HBR patients15
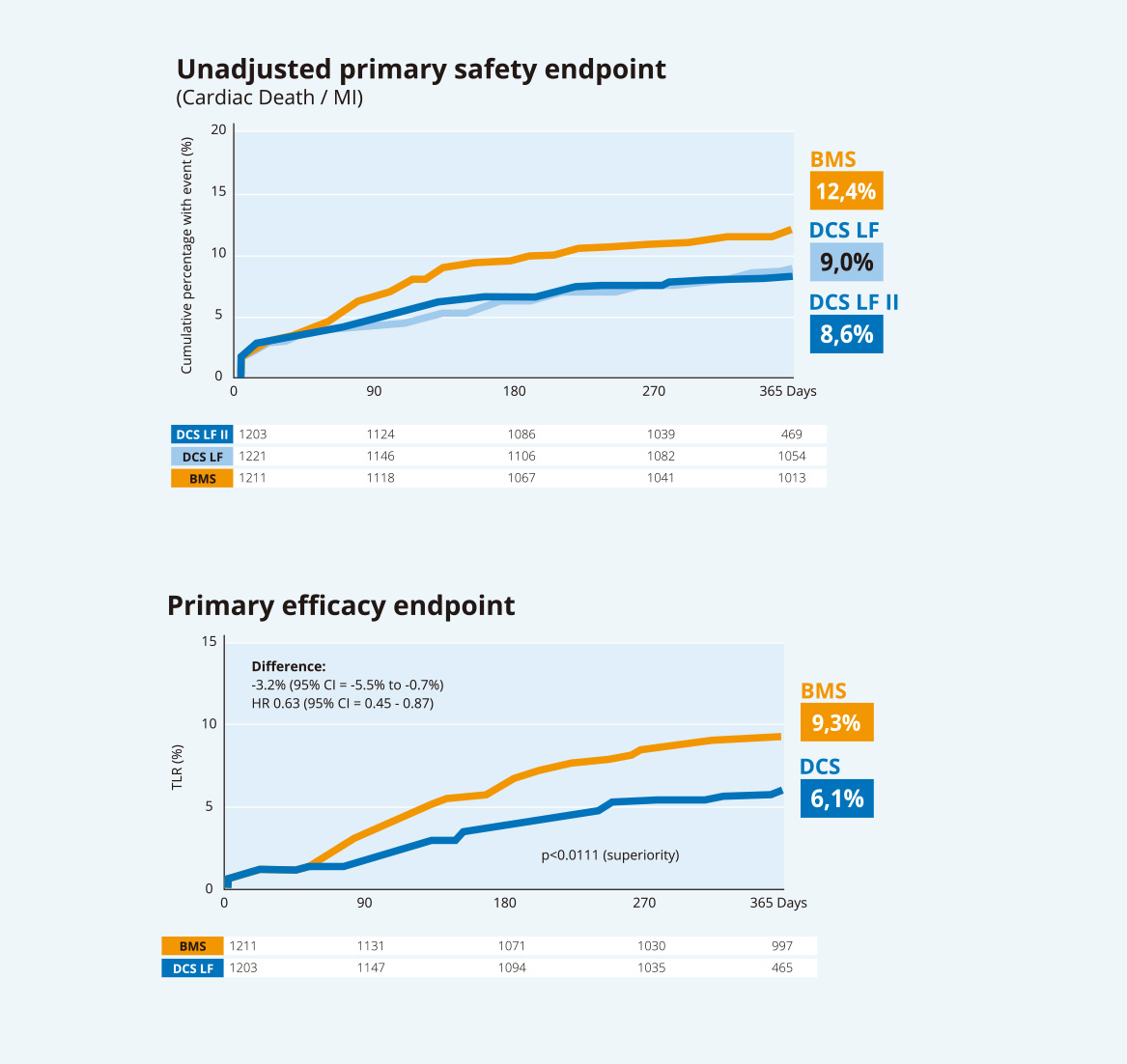
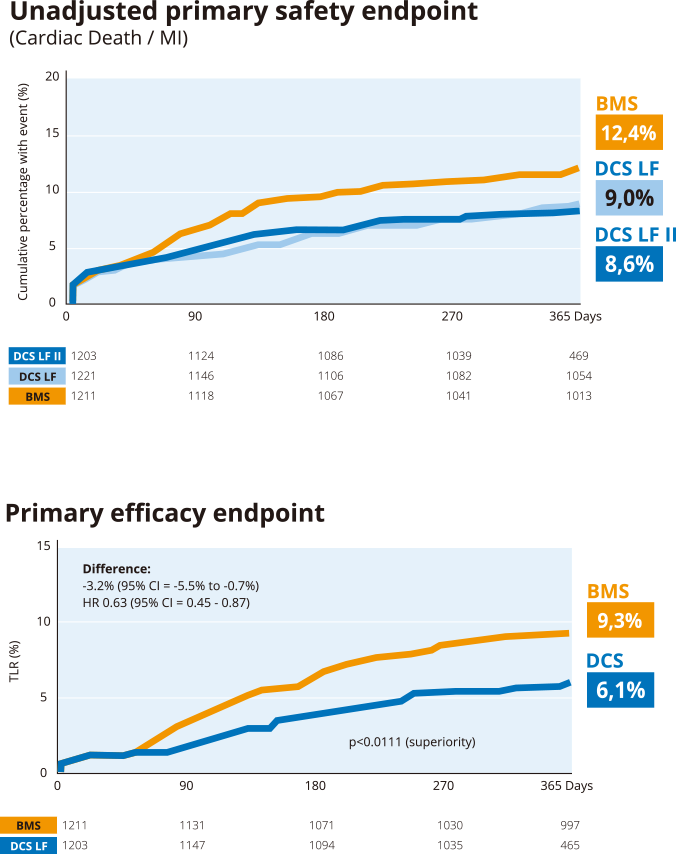
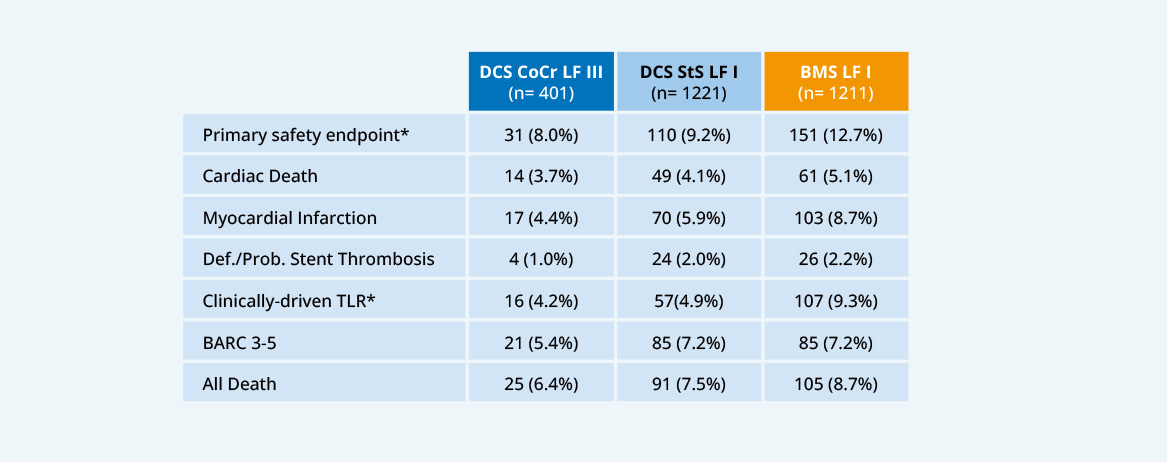
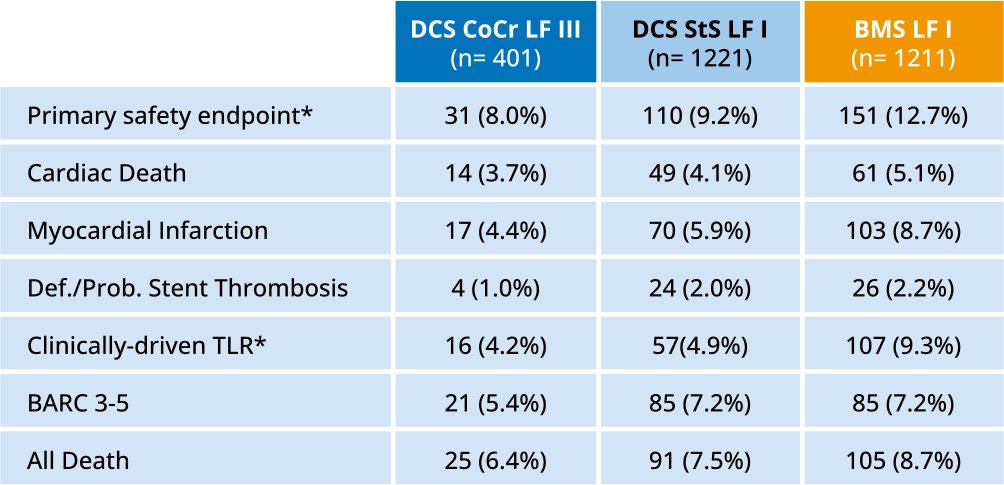
LEADERS FREE III
LEADERS FREE III
- CoCr DCS non-inferior to StS DCS for safety16
- CoCR DCS superior to BMS for efficacy16
![]()
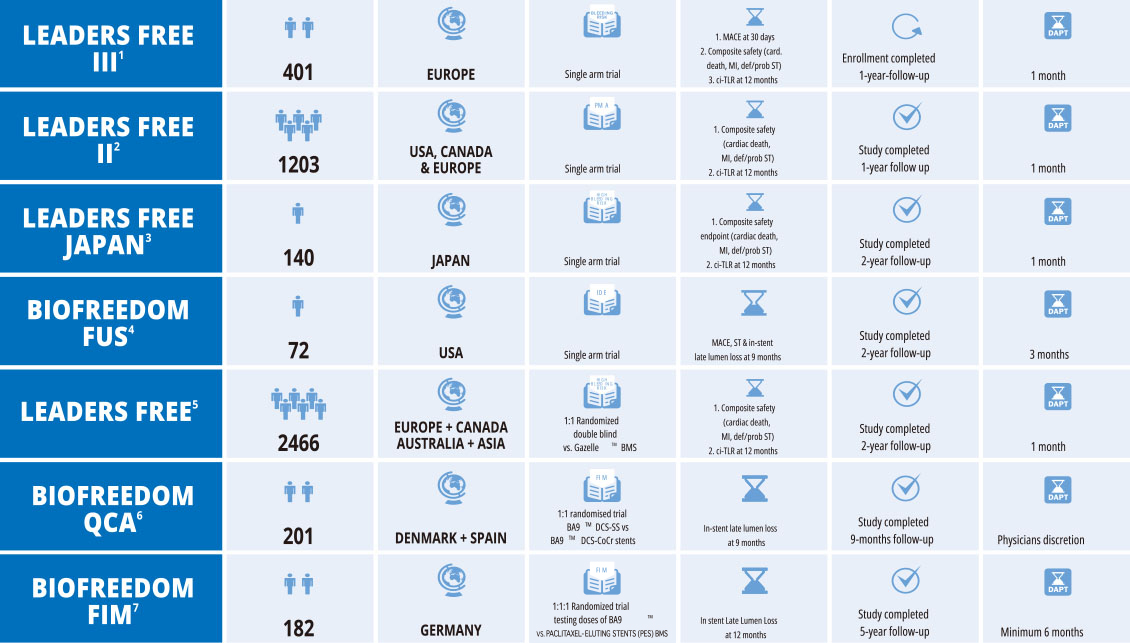
BIOFREEDOM™
FAMILY CLINICAL PROGRAM
BioFreedom™ - Company Managed Trials
11855-000-EN_Rev.03d8
- 1. BioLimus-A9™ coated thin strut stents in High Bleeding Risk patients Oral abstract presentation Eberli R. PCR e-course 2020
- 2. Global Approach to High Bleeding Risk Patients With Polymer-Free Drug-Coated Coronary Stents: The LF II Study. M.W. Krucoff. Circ Cardiovasc Interv. 2020 Apr;13
- 3. ePoster Dr Saito Euro PCR 2017
- 4. Polymer-free Biolimus A9-coated stents in the treatment of de novo coronary lesions with short DAPT: 9-month angiographic and clinical follow-up of the prospective,
multicenter BioFreedom USA clinical trial Waksman R. et al CRM 18 (2017) 475–481 - 5. 2-Year Outcomes of High Bleeding Risk Patients After Polymer-Free Drug-Coated Stents. Garot P et al. JACC VOL.6 9, NO.2 , 2017
- 6. Main results of the BioFreedom QCA trial. Sabate M. PCR e-course 2020
- 7. Polymer-Free Biolimus A9-Coated Stents in the Treatment of De Novo Coronary Lesions 4- and 12-Month Angiographic Follow-Up and Final 5-Year Clinical Outcomes of the Prospective, Multicenter BioFreedom FIM Clinical Trial Costa R et al. J Am Coll Cardiol Intv 2016;9:51–64
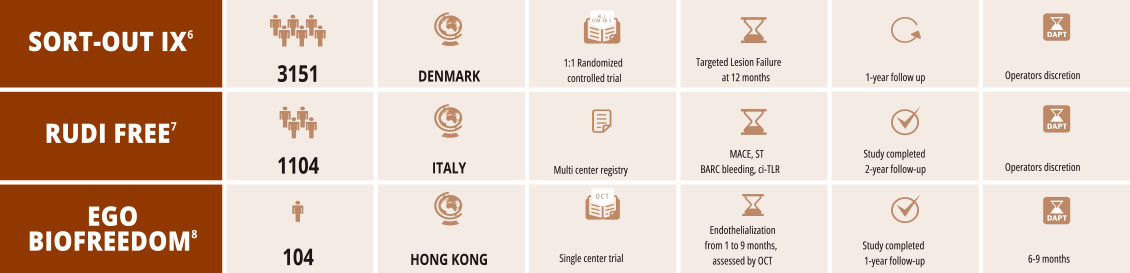
BioFreedom™ - Physician Managed Trials
with support from Biosensors International*
11855-000-EN_Rev.03d8
- * Biosensors has provided financial support for these PITs
- 6. Randomised Comparison of the Polymer-Free Biolimus-Coated BioFreedom Stent with the Ultrathin Strut Biodegradable Polymer Sirolimus-Eluting Stent in an All-comers Population
Treated with Percutaneous Coronary Intervention: The SORT OUT IX Trial. Published online Jensen L. 10.1161/CIRCULATIONAHA.119.0 - 7. Safety and efficacy of polymer-free Biolimus eluting stents in all-comer patients: The RUDI FREE study Sardella G, et al. EuroIn
- 8. Establishment of healing profile and neointimal transformation in the new polymer-free biolimus A9-coated coronary stent by lon
assessments: the EGO-BIOFREEDOM study Lee SWL et al. EuroIntervention. 2018 Sep 20;14(7):780-788
BioFreedom™ - Trials
with no Biosensors International involvement
11855-000-EN_Rev.03d8
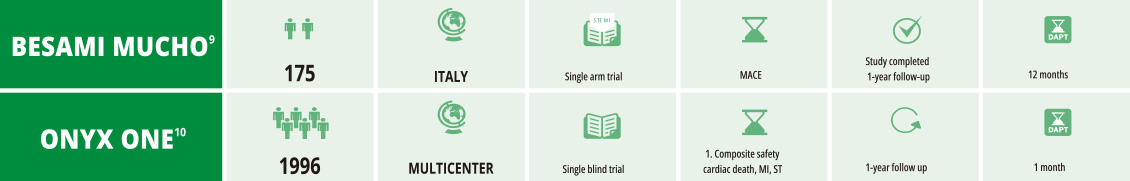
- 9. Angiographic and clinical performance of polymer-free biolimus-eluting stent in patients with ST-segment elevation acute myocardial infarction
in a metropolitan public hospital: The BESAMI MUCHO study Gaspardone A Catheter Cardiovasc Interv.2017;00:1–8 - 10. Polymer-based or Polymer-free Stents in Patients at High Bleeding Risk Windecker S, et al. N Engl J Med. 12th February 2020; 1-11



